

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO 3 (...
Questions

Mathematics, 30.11.2021 01:20




Mathematics, 30.11.2021 01:20

Mathematics, 30.11.2021 01:20


Mathematics, 30.11.2021 01:20



Advanced Placement (AP), 30.11.2021 01:20






Geography, 30.11.2021 01:20

Business, 30.11.2021 01:20


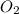 produced = 32 g
produced = 32 g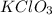 as:-
as:-
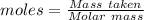
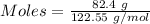
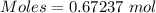
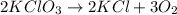
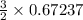 moles of oxygen gas
moles of oxygen gas

