Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
A solution of phosphoric acid was made by dissolving 10.0 g of H3PO4 in 100.0 mL of water. The resul...
Questions

Mathematics, 13.09.2019 02:20



History, 13.09.2019 02:20



Mathematics, 13.09.2019 02:30


Biology, 13.09.2019 02:30


English, 13.09.2019 02:30

Mathematics, 13.09.2019 02:30

Mathematics, 13.09.2019 02:30



History, 13.09.2019 02:30

Biology, 13.09.2019 02:30



History, 13.09.2019 02:30

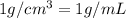
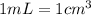
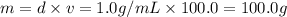
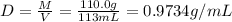
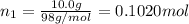
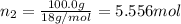
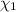
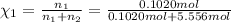
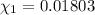
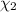
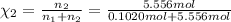
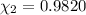
![[Molarity]=\frac{\text{Moles of solute}}{\text{Volume of solution(L)}}](/tpl/images/0549/6266/0dac6.png)
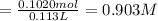
![[Molality]=\frac{\text{Moles of solute}}{\text{Mass of solvent(kg)}}](/tpl/images/0549/6266/71fd2.png)