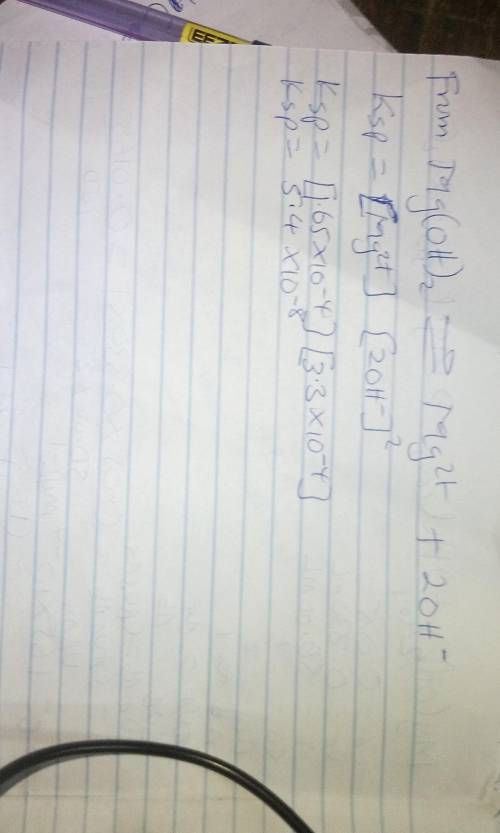Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
In an experiment similar to the one described in this procedure, a saturated solution of Mg(OH)2 was...
Questions

Biology, 18.10.2019 15:30

Biology, 18.10.2019 15:30


Biology, 18.10.2019 15:30

Geography, 18.10.2019 15:30


Physics, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30



World Languages, 18.10.2019 15:30

Biology, 18.10.2019 15:30



Health, 18.10.2019 15:30


Physics, 18.10.2019 15:30

Biology, 18.10.2019 15:30