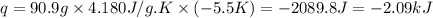Chemistry, 16.03.2020 22:55 brendacauani12345
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1.01g/mL, what is the heat of the calorimeter in kJ given the temperature change of the above equation. You may assume the solution has a heat capacity of 4.180J/gK. Express your final answer in kJ and with 2 decimal places

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2


Chemistry, 23.06.2019 08:40
The half-life of a certain element is 100 days. how many half-lives will it be before only one eighth of this elementremains?
Answers: 1
You know the right answer?
F 65mL of sulfuric acid and 25mL of sodium hydroxide were mixed and the solution had a density of 1....
Questions

Physics, 15.12.2021 14:00

Social Studies, 15.12.2021 14:00






English, 15.12.2021 14:00




Mathematics, 15.12.2021 14:00


English, 15.12.2021 14:00

Advanced Placement (AP), 15.12.2021 14:00

Mathematics, 15.12.2021 14:00


English, 15.12.2021 14:00


History, 15.12.2021 14:00

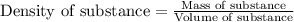
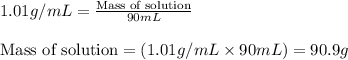
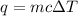
 = change in temperature = -5.5 K
= change in temperature = -5.5 K