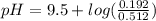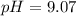Chemistry, 16.03.2020 23:01 thanks5640
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochloric acid are added to 125 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume change does not change upon adding hydrochloric acid)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 23.06.2019 13:30
The two isotopes of chlorine are 3517cl and 3717cl. which isotope is the most abundant?
Answers: 1

You know the right answer?
A buffer solution contains 0.353 M ammonium bromide and 0.352 M ammonia. If 0.0200 moles of hydrochl...
Questions


Biology, 08.03.2021 22:40

English, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40





Mathematics, 08.03.2021 22:40

Mathematics, 08.03.2021 22:40




English, 08.03.2021 22:40



Mathematics, 08.03.2021 22:40


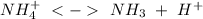
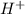 of the hydrochloric acid (
of the hydrochloric acid (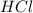 ) will interact with the base of the buffer system (
) will interact with the base of the buffer system (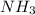 ) to produce more acid (
) to produce more acid (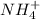 ), so:
), so: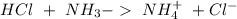
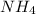 will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So:
will increase. The next step then would be the calculation of the moles of the acid and base in the buffer system. So: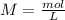
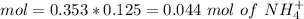
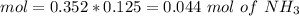
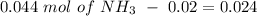
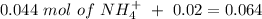
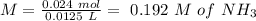
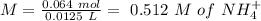
![pH=p{ K }_{ a }+log(\frac { { [A }^{ - }] }{ [HA] } )](/tpl/images/0549/5131/c1f49.png)