
Chemistry, 16.03.2020 22:34 McGregorshamara
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify the state of each reactant and product. + Is iron(III) nitrate considered soluble or not soluble ? ... ... A. Soluble ... B. Not soluble Based upon this, the equilibrium constant for this reaction will be: ... ... A. Greater than 1 ... B. Less than 1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Write the dissolution reaction for iron(III) nitrate in water. Use the pull-down menus to specify th...
Questions

History, 24.10.2021 22:40

Engineering, 24.10.2021 22:40


Mathematics, 24.10.2021 22:40

Mathematics, 24.10.2021 22:40




Chemistry, 24.10.2021 22:40


Mathematics, 24.10.2021 22:40

English, 24.10.2021 22:40

Mathematics, 24.10.2021 22:40


Biology, 24.10.2021 22:40

Mathematics, 24.10.2021 22:40

Computers and Technology, 24.10.2021 22:40


Medicine, 24.10.2021 22:40

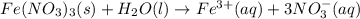
![K_{eq} = [Fe^{3+}][(NO_{3})_{3}]^{3}](/tpl/images/0549/4240/1e6b7.png)


