
Chemistry, 16.03.2020 22:21 lerasteidl
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105 ) b. 0.100 M sodium propanoate (NaC3H5O2) c. pure H2O d. a mixture containing 0.100 M HC3H5O2 and 0.100 M NaC3H5O2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Calculate the pH of each of the following solutions. a. 0.100 M propanoic acid (HC3H5O2, Ka 1.3 105...
Questions






English, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50



Computers and Technology, 11.03.2021 17:50





History, 11.03.2021 17:50


Mathematics, 11.03.2021 17:50

English, 11.03.2021 17:50

![K_{a} = \frac{[C_{3}H_{5}O_{2}^{-}][H_{3}O^{+}]}{[C_{3}H_{6}O_{2}]}](/tpl/images/0549/3755/fa55a.png)
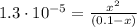 (2)
(2)![pH = -log [H_{3}O^{+}] = -log (0.00113) = 2.95](/tpl/images/0549/3755/8dc10.png)
![K_{b} = \frac{[C_{3}H_{6}O_{2}][OH^{-}]}{[C_{3}H_{5}O_{2}^{-}]}](/tpl/images/0549/3755/eac92.png)
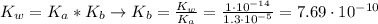
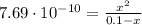 (3)
(3)![pOH = -log[OH^{-}] = -log(8.77\cdot 10^{-6}) = 5.06 \rightarrow pH = 14 - pOH = 8.94](/tpl/images/0549/3755/13708.png)
![K_{w} = [H^{+}][OH^{-}] \rightarrow 1\cdot 10^{-14} = [H^{+}][OH^{-}]](/tpl/images/0549/3755/f80a5.png)
![1\cdot 10^{-14} = [H^{+}]^{2} \rightarrow [H^{+}] = \sqrt{1\cdot 10^{-14}} = 1 \cdot 10^{-7}](/tpl/images/0549/3755/97d56.png)
![pH = -log [H^{+}] = -log (1 \cdot 10^{-7}) = 7.00](/tpl/images/0549/3755/acfd0.png)
![pH = pKa + log(\frac{[C_{3}H_{5}O_{2}^{-}]}{[C_{3}H_{6}O_{2}]}) = -log(1.3 \cdot 10^{-5}) + log(\frac{0.1}{0.1}) = 4.89](/tpl/images/0549/3755/7ff66.png)


