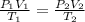A child has a toy balloon with a volume of 1.80 L. The temperature of the balloon when it was filled was 293 K at a pressure of 101.3 kPa. If the child were to let go of the balloon and it rose 3 kilometers into the sky where the pressure of 67.6 kPa and the temperature is 263 K, what would the new volume of the balloon be?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

You know the right answer?
A child has a toy balloon with a volume of 1.80 L. The temperature of the balloon when it was filled...
Questions

Mathematics, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00


Mathematics, 18.09.2021 14:00


Business, 18.09.2021 14:00



Biology, 18.09.2021 14:00



English, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00


Mathematics, 18.09.2021 14:00

English, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00

English, 18.09.2021 14:00