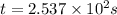Chemistry, 16.03.2020 21:06 josuealejandro9632
Be sure to answer all parts. Acetone is one of the most important solvents in organic chemistry. It is used to dissolve everything from fats and waxes to airplane glue and nail polish. At high temperatures, it decomposes in a first-order process to methane and ketene (CH2═C═O). At 600°C, the rate constant is 8.7 × 10−3 s−1. (a) What is the half-life of the reaction? Give your answer in scientific notation. 7.97 × 10 s (b) How long does it take for 34% of a sample of acetone to decompose? s (c) How long does it take for 89% of a sample of acetone to decompose? Give your answer in scientific notation. × 10 s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 23.06.2019 18:50
Which of the following elements is most likely to have an oxidation state of +2? a. oxygen (0) b. sodium (na) c. chlorine (ci) d. strontium (si)
Answers: 1

Chemistry, 23.06.2019 22:30
Susann makes the following entry in her notebook on friday we are given a blue liquid in a shallow container we place it on the windowsill over the weekend or monday morning there was no liquid left with the dish had some solid blue stuff in it
Answers: 2

You know the right answer?
Be sure to answer all parts. Acetone is one of the most important solvents in organic chemistry. It...
Questions




Physics, 18.03.2021 01:30

English, 18.03.2021 01:30





Mathematics, 18.03.2021 01:30



English, 18.03.2021 01:30


Arts, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


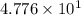 seconds for 34% of a sample of an acetone to decompose.
seconds for 34% of a sample of an acetone to decompose.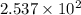 seconds for 89% of a sample of an acetone to decompose.
seconds for 89% of a sample of an acetone to decompose.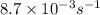
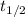
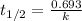
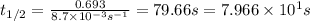
![[A_o]](/tpl/images/0549/1483/dc622.png)
![A=(100\%-34\%)[A_o]=66\%[A_o]=0.66[A_o]](/tpl/images/0549/1483/b7c14.png)
![[A]=[A_o]\times e^{-kt}](/tpl/images/0549/1483/abdec.png)
![0.66[A_o]=[A_o]\times e^{-8.7\times 10^{-3} s^{-1}\times t}](/tpl/images/0549/1483/fef69.png)
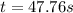
![A=(100\%-89\%)[A-o]=11\%[A_o]=0.11[A_o]](/tpl/images/0549/1483/37410.png)
![0.11[A_o]=[A_o]\times e^{-8.7\times 10^{-3} s^{-1}\times t}](/tpl/images/0549/1483/9ba50.png)