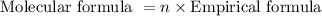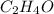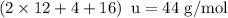Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1
You know the right answer?
The empirical formula of an organic compound is C2H4O. The molecular mass of the compound is 176g/mo...
Questions


Mathematics, 12.10.2021 23:50

Biology, 12.10.2021 23:50


English, 12.10.2021 23:50


History, 12.10.2021 23:50

English, 12.10.2021 23:50

Spanish, 13.10.2021 01:00

English, 13.10.2021 01:00


Mathematics, 13.10.2021 01:00

History, 13.10.2021 01:00


English, 13.10.2021 01:00

Business, 13.10.2021 01:00

History, 13.10.2021 01:00



History, 13.10.2021 01:00

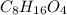 . The molecular formula is obtained by the following expression shown below
. The molecular formula is obtained by the following expression shown below