
Chemistry, 16.03.2020 19:25 mateoperkins
Aluminum is one of the most versatile metals. It is produced by the Hall-Heroult process, in which molten cryolite, Na3AlF6, is used as a solvent for the aluminum ore. Cryolite undergoes very slight decomposition with heat to produce a tiny amount of F2, which escapes into the atmosphere above the solvent. Kc is 2 10-104 at 1300 K for the reaction. Na3AlF6(l) 3 Na(l) Al(l) 3 F2(g) What is the concentration of F2 over a bath of molten cryolite at this temperature

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Aluminum is one of the most versatile metals. It is produced by the Hall-Heroult process, in which m...
Questions

Mathematics, 19.03.2021 03:50

English, 19.03.2021 03:50



Spanish, 19.03.2021 03:50



Mathematics, 19.03.2021 03:50





Mathematics, 19.03.2021 03:50

Mathematics, 19.03.2021 03:50



Social Studies, 19.03.2021 03:50



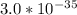
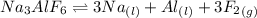
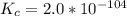
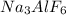
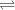
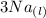

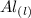
![K_c = [x]^3](/tpl/images/0548/8902/65ef1.png)
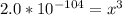
![x =\sqrt[3]{2.0*10^{-104}}](/tpl/images/0548/8902/b2361.png)
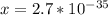
 ≅
≅ 


