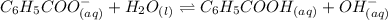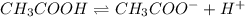Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definitions for acids and bases.
1) An Arrhenius acid is a substance that dissolves in water to produce H+ or H3O+.
2) A Br∅nsted-Lowry base is a proton acceptor.
3) CH3COOH is an Arrhenius base.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1


You know the right answer?
Determine if each statement below is True or False regarding Arrhenius and Br∅nsted-Lowry definition...
Questions












Mathematics, 13.11.2019 04:31




Mathematics, 13.11.2019 04:31

Computers and Technology, 13.11.2019 04:31

Chemistry, 13.11.2019 04:31


 .
.