
Chemistry, 16.03.2020 18:59 nicollexo21
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phosphoric acid and the sodium salts NaH2PO4, Na2HPO4, and Na3PO4 available. (Enter all numerical answers to three significant figures.)
H3PO4(s) + H2O(l) <> H3O+(aq) + H2PO4−(aq) Ka1 = 6.9 ✕ 10−3
H2PO4−(aq) + H2O(l) <> H3O+(aq) + HPO42−(aq) Ka2 = 6.2 ✕ 10−8
HPO42−(aq) + H2O(l) <> H3O+(aq) + PO43−(aq) Ka3 = 4.8 ✕ 10−13
Which of the available chemicals will you use for the acid component of your buffer?
A. H3PO4
B. NaH2PO4
C. Na2HPO4
D. Na3PO4
Which of the available chemicals will you use for the base component of your buffer?
A. H3PO4B. NaH2PO4C. Na2HPO4D. Na3PO4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
You are instructed to create 900. mL of a 0.29 M phosphate buffer with a pH of 7.8. You have phospho...
Questions

Mathematics, 08.06.2021 14:00





History, 08.06.2021 14:00

Chemistry, 08.06.2021 14:00


Law, 08.06.2021 14:00

English, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

English, 08.06.2021 14:00


Chemistry, 08.06.2021 14:00

Mathematics, 08.06.2021 14:00

English, 08.06.2021 14:00

Physics, 08.06.2021 14:00

Business, 08.06.2021 14:00

English, 08.06.2021 14:00

![pKa_1=-Log[6.9x10^-^3]=2.16](/tpl/images/0548/8384/6a482.png)
![pKa_2=-Log[6.2x10^-^8]=7.20](/tpl/images/0548/8384/8337b.png)
![pKa_3=-Log[4.8x10^-^13]=12.31](/tpl/images/0548/8384/78632.png)
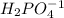 is the acid and
is the acid and 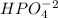 is the base. Therefore the answer for the first question is B and the answer for the second question is C.
is the base. Therefore the answer for the first question is B and the answer for the second question is C.


