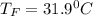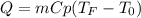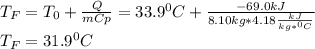Chemistry, 16.03.2020 18:59 cameron12502
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8.10kg of water at 33.9 degrees celsius . During the reaction 69.0kJ of heat flows out of the bath and into the flask.
Required:
Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J*g*K. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 8....
Questions


Mathematics, 12.09.2019 22:30







Mathematics, 12.09.2019 22:30


History, 12.09.2019 22:30


History, 12.09.2019 22:30



Mathematics, 12.09.2019 22:30


Mathematics, 12.09.2019 22:30

Mathematics, 12.09.2019 22:30