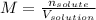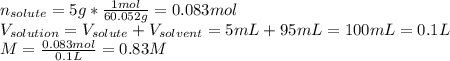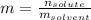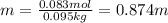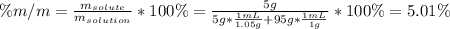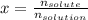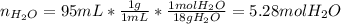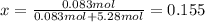Chemistry, 16.03.2020 18:22 cupcake122016
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density of acetic acid is 1.05 g/ml and water is 1.00 g/ml. from this data calculate the concentration of acetic acid in vinegar in: molality, molarity, parts by mass, and the mole fraction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

You know the right answer?
A bottle of commercial vinegar contains 5% acetic acid, ch3cooh, by volume (95% water). the density...
Questions

Mathematics, 26.01.2021 06:40


Mathematics, 26.01.2021 06:40

Mathematics, 26.01.2021 06:40

Biology, 26.01.2021 06:40


Physics, 26.01.2021 06:40


English, 26.01.2021 06:40





Mathematics, 26.01.2021 06:40

Mathematics, 26.01.2021 06:40


Business, 26.01.2021 06:40


English, 26.01.2021 06:40

History, 26.01.2021 06:40