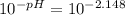Chemistry, 16.03.2020 18:08 jasminer257
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration was found to be 4.215. If the initial pH of the weak acid solution (before titration) has a pH of 2.148, what was the concentration of the weak acid solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
N unknown weak acid, HA, it titrated with 0.6 M NaOH. The pH at the halfway point of this titration...
Questions


History, 27.05.2021 16:20



Mathematics, 27.05.2021 16:20

Mathematics, 27.05.2021 16:20

Mathematics, 27.05.2021 16:20

Mathematics, 27.05.2021 16:20




Mathematics, 27.05.2021 16:20

Social Studies, 27.05.2021 16:20


Health, 27.05.2021 16:20

Mathematics, 27.05.2021 16:20



English, 27.05.2021 16:20

Business, 27.05.2021 16:20

 M
M