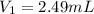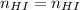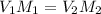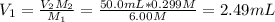Chemistry, 16.03.2020 18:09 TerronRice
You wish to make a 0.299 M hydroiodic acid solution from a stock solution of 6.00 M hydroiodic acid. How much concentrated acid must you add to obtain a total volume of 50.0 mL of the dilute solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 16:00
Water is called the universal solvent because more substances dissolve in water than in any other chemical. this has to do with the polarity of each water molecule. the hydrogen side of each water (h2o) molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge.
Answers: 3
You know the right answer?
You wish to make a 0.299 M hydroiodic acid solution from a stock solution of 6.00 M hydroiodic acid....
Questions

Mathematics, 30.01.2020 07:58


Mathematics, 30.01.2020 07:58

Mathematics, 30.01.2020 07:58

English, 30.01.2020 07:58

Mathematics, 30.01.2020 07:58

Mathematics, 30.01.2020 07:58


Mathematics, 30.01.2020 07:58

English, 30.01.2020 07:58


Business, 30.01.2020 07:58



Spanish, 30.01.2020 07:58

Mathematics, 30.01.2020 07:58

Mathematics, 30.01.2020 07:58



Mathematics, 30.01.2020 07:58