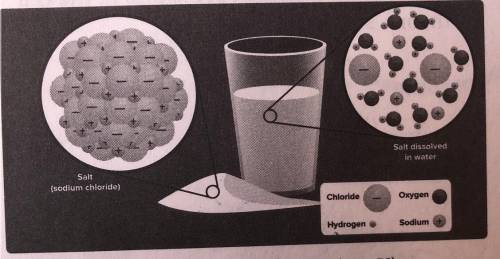
1. The picture above shows salt (NaCl).
dissolved in water (H, O). Which statement
is tr...

1. The picture above shows salt (NaCl).
dissolved in water (H, O). Which statement
is true? SC.6.N.1.1
A Chloride is attracted to the hydrogen atoms in a water molecule.
B Sodium is attracted to the hydrogen atoms in a water molecule.
C Neither sodium nor chloride is attracted to the atoms in a water molecule.
D Sodium and chloride are attracted to the oxygen atoms in a water molecule.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
The electron configurations of two different atoms are shown below. each yellow electron has a charge of 1−, and the net charge of each nucleus is shown. these atoms will combine with bond. a. an ionic b. a positive c. a negative d. a covalent plzzz mee with !
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Questions


Mathematics, 27.07.2021 06:00

Mathematics, 27.07.2021 06:00

Chemistry, 27.07.2021 06:00

Spanish, 27.07.2021 06:00


Mathematics, 27.07.2021 06:00

Social Studies, 27.07.2021 06:00

Mathematics, 27.07.2021 06:00

English, 27.07.2021 06:00

English, 27.07.2021 06:00



English, 27.07.2021 06:00


Mathematics, 27.07.2021 06:00






