
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. You determine that the concentration of the unknown acid was 0.1224 M. You also determined that the acid was monoprotic (HA). What is the K_a and pK_a of your unknown acid

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
You are given 10.00 mL of a solution of an unknown acid. The pH of this solution is exactly 2.95. Yo...
Questions

Computers and Technology, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Chemistry, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

Physics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

English, 22.10.2020 02:01

Chemistry, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01


English, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01

 is dissociation constant and the value of
is dissociation constant and the value of 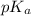 is 4.98.
is 4.98.![pH=-\log[H^+]](/tpl/images/0548/6523/cf945.png)
![2.95=-\log[H^+]](/tpl/images/0548/6523/b4bb5.png)
![[H^+]=10^{-2.95}=0.001122 M](/tpl/images/0548/6523/2ad17.png) ..[1]
..[1]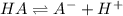
![K_a=\frac{[A^-][H^+]}{[HA]}](/tpl/images/0548/6523/a5cb9.png)
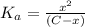
![[H^+]=x =0.001122 M](/tpl/images/0548/6523/90707.png) ( from [1])
( from [1])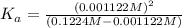
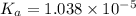
![pK_a=-\log[K_a]](/tpl/images/0548/6523/78bbf.png)
![=-\log[1.038\times 10^{-5}]=4.98](/tpl/images/0548/6523/44380.png)


