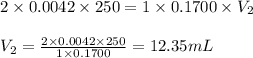Chemistry, 16.03.2020 16:37 DonovanBaily42
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of volumetric flask and dilutes to the mark with distilled water. She plans to titrate the acid with 0.1700 M NaOH solution. Calculate the volume of NaOH solution in milliliters the student will need to add to reach the final equivalence point. (Molar mass of sulfurous acid = 82.079 g/moL)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
A chemist weighs out 0.0865 g of sulfurous acid, H2SO3, which is a diprotic acid into a 250. mL of v...
Questions

Mathematics, 22.10.2019 03:30




Chemistry, 22.10.2019 03:30

History, 22.10.2019 03:30


Mathematics, 22.10.2019 03:30


Mathematics, 22.10.2019 03:30






Mathematics, 22.10.2019 03:30

Mathematics, 22.10.2019 03:30


Mathematics, 22.10.2019 03:30

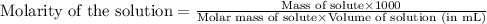

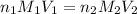
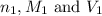 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 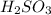
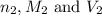 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.