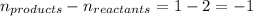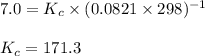The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g...

Chemistry, 16.03.2020 16:11 maddie53116
The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g) ⇌ N2O4(g) Kp = 7.0
If a sealed flask contains 1.5 atm of NO2 and 14.2 atm of N2O4. Calculate the value of Kc for the reaction,

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

You know the right answer?
Questions

English, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00



Biology, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00



Arts, 05.03.2021 01:00

History, 05.03.2021 01:00


Chemistry, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00


Arts, 05.03.2021 01:00

Biology, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00

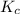 for given reaction is 171.3
for given reaction is 171.3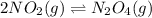
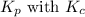 is given by the formula:
is given by the formula: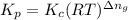
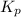 = equilibrium constant in terms of partial pressure = 7.0
= equilibrium constant in terms of partial pressure = 7.0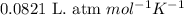
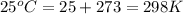
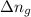 = change in number of moles of gas particles =
= change in number of moles of gas particles =