
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your work. P4 + NO3–→ H2PO4– + NO(A) What is the oxidized element? What is the reduced element?
(B) Write balanced half reactions for the oxidation reaction:
(C) Write balanced half reactions for the reduction reaction:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 17:40
Which type of bonding involves the complete transfer of a valence electron from a less electronegative atom to a more electronegative one?
Answers: 1
You know the right answer?
Balance the following chemical equation by the half-reaction method in acidic conditions. Show your...
Questions










Mathematics, 13.01.2021 17:20









Arts, 13.01.2021 17:20

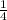 P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e
P4 + 4H2O ⇒ H2PO4- + 6H+ + 5e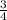 P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-
P4 + 5NO3- +2H+ + 2H2O⇒ 5NO+3H3PO4-

