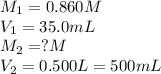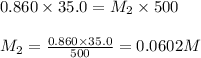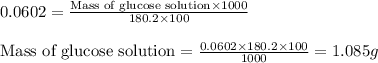A student placed 15.5 g of glucose (C6H12O6) in a volumetric flask, added enough water to dissolve the glucose by swirling, then carefully added additional water until the 100. mL mark on the neck of the flask was reached. The flask was then shaken until the solution was uniform. A 35.0 mL sample of this glucose solution was diluted to 0.500 L. How many grams of glucose are in 100. mL of the final solution?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
A student placed 15.5 g of glucose (C6H12O6) in a volumetric flask, added enough water to dissolve t...
Questions

English, 31.01.2020 13:51

Mathematics, 31.01.2020 13:51



Mathematics, 31.01.2020 13:51


Mathematics, 31.01.2020 13:51


Advanced Placement (AP), 31.01.2020 13:51

History, 31.01.2020 13:51


Social Studies, 31.01.2020 13:51



Mathematics, 31.01.2020 13:51





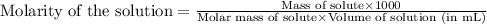 ......(1)
......(1)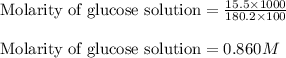
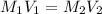
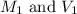 are the molarity and volume of the concentrated glucose solution
are the molarity and volume of the concentrated glucose solution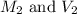 are the molarity and volume of diluted glucose solution
are the molarity and volume of diluted glucose solution