PLZ HELP, GIVING BRAINLIEST!!
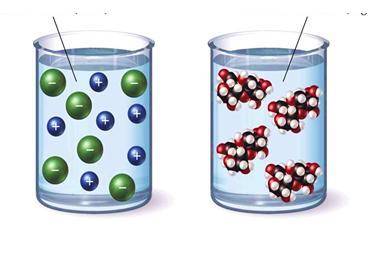
In class, students were given the pictures below and asked to pi...

Chemistry, 14.03.2020 00:29 cxttiemsp021
PLZ HELP, GIVING BRAINLIEST!!
In class, students were given the pictures below and asked to pick a solution that would conduct electricity and to justify their choice.
Based on the model above, which student's argument is correct?
A. Student B claims that the left beaker contains a covalent compound because the solute breaks apart into charged particles.
B. Student D claims to identify the solute as either ionic or covalent more information is needed than what is provided in the model.
C. Student C claims that the right beaker contains an ionic compound because the solute stays together when dissolved.
D. Student A claims that the left beaker contains an ionic compound because the solute breaks apart into charged particles.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2


Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Questions

History, 02.12.2020 02:40


Arts, 02.12.2020 02:40




Mathematics, 02.12.2020 02:40





Mathematics, 02.12.2020 02:40


Mathematics, 02.12.2020 02:40


Mathematics, 02.12.2020 02:40






