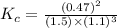Chemistry, 13.03.2020 23:58 gwendallinesikes
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [N2]eq = 1.5 M, [H2]eq = 1.1 M, [NH3]eq = 0.47 M. N2(g) + 3 H2(g) 2 NH3(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1


Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follow...
Questions

History, 28.08.2019 03:30

History, 28.08.2019 03:30



Mathematics, 28.08.2019 03:30

Biology, 28.08.2019 03:30


Mathematics, 28.08.2019 03:30




Mathematics, 28.08.2019 03:30

History, 28.08.2019 03:30


Mathematics, 28.08.2019 03:30


Biology, 28.08.2019 03:30

Mathematics, 28.08.2019 03:30

History, 28.08.2019 03:30

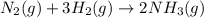
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0547/2110/c3aa0.png)