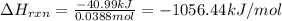Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
If 1.785 g of ethanol (CHCHOH) is burned in a constant volume calorimeter causing a temperature incr...
Questions

English, 28.01.2020 05:31


History, 28.01.2020 05:31





Mathematics, 28.01.2020 05:31

English, 28.01.2020 05:31


Physics, 28.01.2020 05:31

Social Studies, 28.01.2020 05:31

Biology, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31


History, 28.01.2020 05:31

Advanced Placement (AP), 28.01.2020 05:31


Social Studies, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31

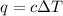
 = change in temperature = 4.32°C
= change in temperature = 4.32°C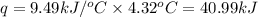
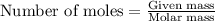
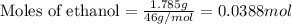
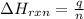
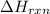 = enthalpy change of the reaction
= enthalpy change of the reaction