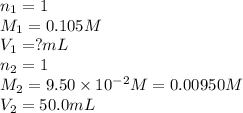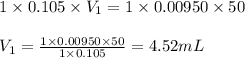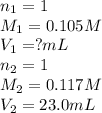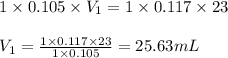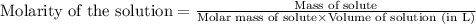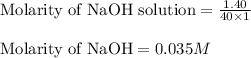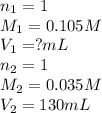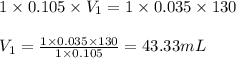Chemistry, 13.03.2020 22:21 WritingStar1313
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equivalence point?
a. 50.0 mL of 9.5010?2 M NaOH
b. 23.0 mL of 0.117 M NH3
c. 130 mL of a solution that contains 1.40 g of NaOH per liter

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equ...
Questions

Mathematics, 10.12.2020 14:50




Mathematics, 10.12.2020 14:50






Mathematics, 10.12.2020 14:50

Mathematics, 10.12.2020 14:50


English, 10.12.2020 14:50


Biology, 10.12.2020 14:50


Chemistry, 10.12.2020 14:50

English, 10.12.2020 14:50

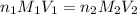
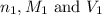 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid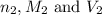 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base