
Chemistry, 27.08.2019 13:30 chumreaper2002
Consider the positions of carbon, nitrogen, potassium, and calcium on the periodic table. the atoms of which element attract electrons most strongly in chemical bonds? carbon (c) calcium (ca) nitrogen (n) potassium (k)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Consider the positions of carbon, nitrogen, potassium, and calcium on the periodic table. the atoms...
Questions

Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00


Mathematics, 26.04.2021 22:00


Advanced Placement (AP), 26.04.2021 22:00






Physics, 26.04.2021 22:00



Mathematics, 26.04.2021 22:00

Mathematics, 26.04.2021 22:00




Biology, 26.04.2021 22:00

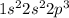 .
.

