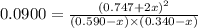Chemistry, 13.03.2020 19:56 donttrip10
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C . H 2 O ( g ) + Cl 2 O ( g ) − ⇀ ↽ − 2 HOCl ( g ) K c = 0.0900 at 25 ° C Calculate the equilibrium concentrations of each gas at 25 ° C .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C ....
Questions

Biology, 04.11.2020 17:00

Mathematics, 04.11.2020 17:00





Mathematics, 04.11.2020 17:00

Arts, 04.11.2020 17:00


Spanish, 04.11.2020 17:00


Social Studies, 04.11.2020 17:00

Mathematics, 04.11.2020 17:00

English, 04.11.2020 17:00

History, 04.11.2020 17:00





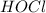 ,
, 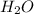 and
and 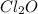 at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.
at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.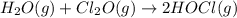
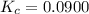
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0546/8405/da783.png)