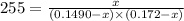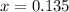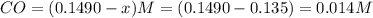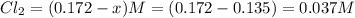CO (g) + Cl2 (g) ⇌ COCl2 (g)

Chemistry, 13.03.2020 19:17 owenbarrows
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
A reaction mixture initially contains a CO concentration of 0.1490 M and a Cl2 concentration of 0.172 M at 1000 K.
Part A
What is the equilibrium concentration of CO at 1000 K?
Express your answer in molarity to three significant figures.
Part B
What is the equilibrium concentration of Cl2 at 1000 KK?
Express your answer in molarity to three significant figures.
Part C
What is the equilibrium concentration of COCl2 at 1000 KK?
Express your answer in molarity to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
CO (g) + Cl2 (g) ⇌ COCl2 (g)
Questions

Biology, 21.06.2019 15:30

Mathematics, 21.06.2019 15:30




Physics, 21.06.2019 15:30


Social Studies, 21.06.2019 15:30


World Languages, 21.06.2019 15:30


Arts, 21.06.2019 15:30








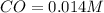
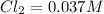

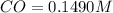
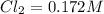
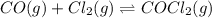
![K_c=\frac{[COCl_2]}{[Cl_2]\times [CO]}](/tpl/images/0546/7266/b0e05.png)