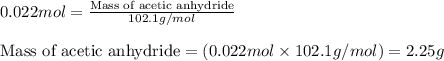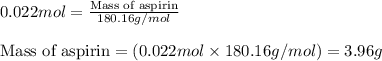Chemistry, 13.03.2020 18:34 chloeann5397
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according to the following equation:
C7H6O3Salicylicacid+C4H6O3Aceticanh ydride→C9H8O4Aspirin+CH3COOHAcetica cid
a. How many grams of acetic anhydride are needed to react with 3.07 g of salicylic acid?
b. How many grams of aspirin will result?
c. How many grams of acetic acid are formed as a by-product?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according t...
Questions






Mathematics, 18.03.2021 03:30

Social Studies, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Biology, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Biology, 18.03.2021 03:30




Mathematics, 18.03.2021 03:30



Social Studies, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

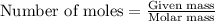 .....(1)
.....(1)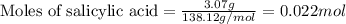
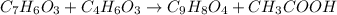
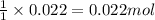 of acetic anhydride
of acetic anhydride