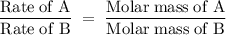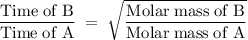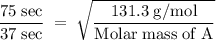Chemistry, 13.03.2020 06:01 kiarabermudez754
A sample of Xe takes 75 seconds to effuse out of a container. An unknown gas takes 37 seconds to effuse out of the identical container under identical conditions. What is the most likely identity of the unknown gas?
a. Br2
b. He
c. O2
d. Kr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
A sample of Xe takes 75 seconds to effuse out of a container. An unknown gas takes 37 seconds to eff...
Questions

Mathematics, 07.12.2020 18:40




Mathematics, 07.12.2020 18:40


Mathematics, 07.12.2020 18:40

Engineering, 07.12.2020 18:40







Mathematics, 07.12.2020 18:40

Geography, 07.12.2020 18:40

History, 07.12.2020 18:40

Biology, 07.12.2020 18:40


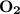 . Thus, option C is correct.
. Thus, option C is correct.