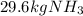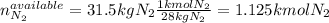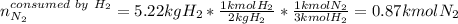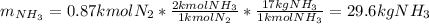Chemistry, 13.03.2020 02:27 ddddre3909
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kg, that we can synthesize from 5.22 kg of H2 and 31.5 kg of N2? Express the mass in kilograms to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield...
Questions


Health, 20.07.2019 03:30

History, 20.07.2019 03:30

Engineering, 20.07.2019 03:30



Mathematics, 20.07.2019 03:30

Computers and Technology, 20.07.2019 03:30


Health, 20.07.2019 03:30


History, 20.07.2019 03:30

Geography, 20.07.2019 03:30






Chemistry, 20.07.2019 03:30

Computers and Technology, 20.07.2019 03:30