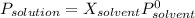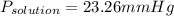Chemistry, 12.03.2020 18:28 EmmaKozlewski4907
If the van't Hoff factor for LiCl in a 0.62m solution is 1.92, what is the vapor pressure depression in mmHg of the solution at 298 K? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
If the van't Hoff factor for LiCl in a 0.62m solution is 1.92, what is the vapor pressure depression...
Questions


Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Physics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Computers and Technology, 16.08.2020 01:01

Business, 16.08.2020 01:01

English, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01

Social Studies, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Advanced Placement (AP), 16.08.2020 01:01

Mathematics, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01