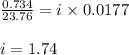Chemistry, 12.03.2020 17:25 aubreymoore4553
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressure depression of 0.734 mmHg at 298 ∘C? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressur...
Questions

Mathematics, 06.05.2020 15:01

Chemistry, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01



Spanish, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01




Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01


Biology, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

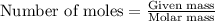
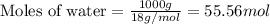
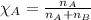
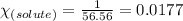
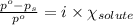
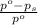 = relative lowering in vapor pressure = 0.734 mmHg
= relative lowering in vapor pressure = 0.734 mmHg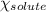 = mole fraction of solute = 0.0177
= mole fraction of solute = 0.0177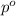 = vapor pressure of pure water = 23.76 torr
= vapor pressure of pure water = 23.76 torr