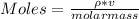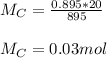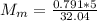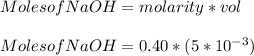You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of moles of vegetable oil, methanol, and NaOH that are initially present in the sample. Assume the density of vegetable oil is 0.895 g/mL and the molar mass is 895 g/mol. Look up the density and molar mass of any other compounds as needed.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1

Chemistry, 23.06.2019 11:50
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1
You know the right answer?
You added 5.00 mL of 0.40 M NaOH in methanol to 20.00 mL of cooking oil. Calculate the number of mol...
Questions




Mathematics, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00


Chemistry, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00



Geography, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00

Chemistry, 28.04.2021 01:00

Biology, 28.04.2021 01:00