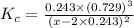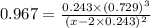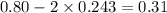The equilibrium constant, Kc, for the following reaction is 0.967 at 650 K. 2NH3(g) N2(g) 3H2(g) When a sufficiently large sample of NH3(g) is introduced into an evacuated vessel at 650 K, the equilibrium concentration of H2(g) is found to be 0.729 M. Calculate the concentration of NH3 in the equilibrium mixture. M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 0.967 at 650 K. 2NH3(g) N2(g) 3H2(g) Whe...
Questions

Mathematics, 10.05.2021 21:30

Physics, 10.05.2021 21:30


English, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30


Mathematics, 10.05.2021 21:30


English, 10.05.2021 21:30

History, 10.05.2021 21:30


Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30


Chemistry, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Mathematics, 10.05.2021 21:30

Geography, 10.05.2021 21:30

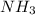 in the equilibrium mixture is 0.31 M
in the equilibrium mixture is 0.31 M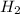 = 0.729 M
= 0.729 M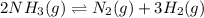
![K_c=\frac{[y]\times [3y]^3}{[x-2y]^2}](/tpl/images/0544/6710/3f7db.png)