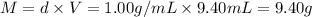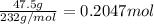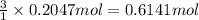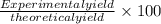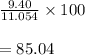Chemistry, 12.03.2020 05:32 kfcnkfnmnfk9513
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess hydrogen gas to produce metallic tungsten and 9.40 mL water (d = 1.00 g/mL)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
What is the percent yield of a reaction in which 47.5 g tungsten (VI) oxide (WO3) reacts with excess...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








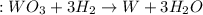
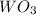 = 47.5g
= 47.5g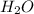 = 18 g/mole
= 18 g/mole