

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
Determine the number of grams of C4H10 that are required to completely react to produce 8.70 mol of...
Questions

Mathematics, 17.04.2020 01:28








History, 17.04.2020 01:29







Social Studies, 17.04.2020 01:29

English, 17.04.2020 01:29

Mathematics, 17.04.2020 01:29

Computers and Technology, 17.04.2020 01:29

English, 17.04.2020 01:29

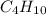 are required
are required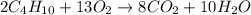
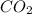 are produced from 2 moles of
are produced from 2 moles of 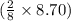 moles of
moles of 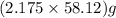 = 126.4 g
= 126.4 g

