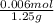Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
It takes 12.45 milliliters of 0.50 M NAOH to back titrate a mixture containing 1.25 grams of baking...
Questions

Mathematics, 05.02.2022 18:50




Mathematics, 05.02.2022 18:50

Mathematics, 05.02.2022 18:50



Social Studies, 05.02.2022 18:50


History, 05.02.2022 18:50







English, 05.02.2022 18:50

History, 05.02.2022 18:50

 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)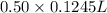 (12.45 ml = 0.1245 L)
(12.45 ml = 0.1245 L)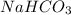 will be as follows.
will be as follows.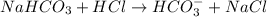
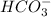 . Hence, moles of
. Hence, moles of 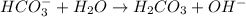
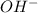 .
.