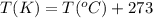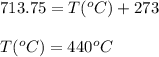Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1


You know the right answer?
A sample of gas has a volume of 1.24 L under 2.35 atm pressure at 45°C. If the gas is then expanded...
Questions


Mathematics, 17.12.2020 01:00



Mathematics, 17.12.2020 01:00


Advanced Placement (AP), 17.12.2020 01:00



Advanced Placement (AP), 17.12.2020 01:00


Physics, 17.12.2020 01:00



History, 17.12.2020 01:00



Chemistry, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

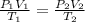
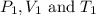 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas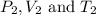 are the final pressure, volume and temperature of the gasW
are the final pressure, volume and temperature of the gasW![P_1=2.35atm\\V_1=1.24L\\T_1=45^oC=[45+273]K=318K\\P_2=0.515atm\\V_2=12.7L\\T_2=?](/tpl/images/0544/2731/0dccc.png)