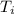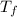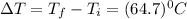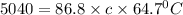Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

You know the right answer?
An unknown metal has a mass of 86.8 g. When 5040 J of heat are added to the sample, the sample tempe...
Questions

History, 02.10.2019 13:20

Mathematics, 02.10.2019 13:20

Mathematics, 02.10.2019 13:20

English, 02.10.2019 13:20


Mathematics, 02.10.2019 13:20

Chemistry, 02.10.2019 13:20

Mathematics, 02.10.2019 13:20

Social Studies, 02.10.2019 13:20

Social Studies, 02.10.2019 13:20


Physics, 02.10.2019 13:20

Social Studies, 02.10.2019 13:20

Chemistry, 02.10.2019 13:20



Social Studies, 02.10.2019 13:20

Mathematics, 02.10.2019 13:20


Mathematics, 02.10.2019 13:20

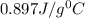
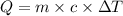
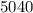 Joules
Joules