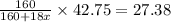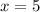Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1


Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
A chemist is given a sample of the CuSO4 hydrate and asked to determine its the empirical formula. T...
Questions

Biology, 26.03.2021 18:30

Geography, 26.03.2021 18:30


English, 26.03.2021 18:30

Mathematics, 26.03.2021 18:30


Chemistry, 26.03.2021 18:30

Chemistry, 26.03.2021 18:30

Mathematics, 26.03.2021 18:30



Mathematics, 26.03.2021 18:30


English, 26.03.2021 18:30



French, 26.03.2021 18:30

Biology, 26.03.2021 18:30



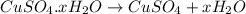
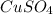 = 160 g/mol
= 160 g/mol decomposes to give 160 g of anhydrous
decomposes to give 160 g of anhydrous 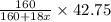 g of anhydrous
g of anhydrous