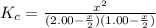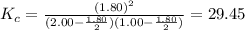Chemistry, 12.03.2020 02:05 martinbricein10
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(g). At equilibrium, it is found that 1.80 moles of HI(g) are present in the container. Calculate K for the reaction: H2(g) + I2(g) ⇄ 2 HI(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(...
Questions


Physics, 25.06.2019 20:00




Mathematics, 25.06.2019 20:00

History, 25.06.2019 20:00




Social Studies, 25.06.2019 20:00


Mathematics, 25.06.2019 20:00


History, 25.06.2019 20:00


Physics, 25.06.2019 20:00



History, 25.06.2019 20:00

![[H_2]= \frac{2.00 mol}{1.00 L}=2.00 M](/tpl/images/0544/0690/a78aa.png)
![[I_2]= \frac{I.00 mol}{1.00 L}=1.00 M](/tpl/images/0544/0690/94c40.png)
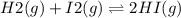
![[HI]=\frac{1.80 mol}{1.00L} = 1.80M= x](/tpl/images/0544/0690/bcad7.png)
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0544/0690/62646.png)