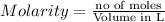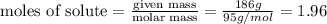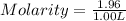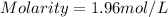Chemistry, 12.03.2020 00:33 candancejc557
When a 1.00 L sample of water from the surface of the Dead Sea (which is more than 400 meters below sea level and much saltier than ordinary seawater) is evaporated, 186 grams of MgCl2 are recovered. What is the molarity of MgCl2 in the original sample? M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
When a 1.00 L sample of water from the surface of the Dead Sea (which is more than 400 meters below...
Questions

Mathematics, 03.03.2020 17:31




Biology, 03.03.2020 17:31











Biology, 03.03.2020 17:31

Biology, 03.03.2020 17:31



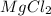 in the original sample was 1.96M
in the original sample was 1.96M