
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 67.7 g of sulfuric acid is mixed with 83. g of sodium hydroxide. Calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions

Mathematics, 20.04.2020 21:05

Mathematics, 20.04.2020 21:05


Mathematics, 20.04.2020 21:05




Mathematics, 20.04.2020 21:05

Mathematics, 20.04.2020 21:05

Mathematics, 20.04.2020 21:05






History, 20.04.2020 21:05




Mathematics, 20.04.2020 21:05

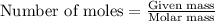
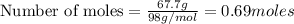
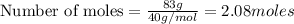
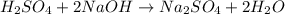
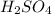 require 2 moles of
require 2 moles of 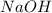
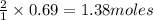 of
of 

