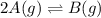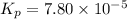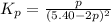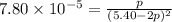Consider the reaction.
2A(g)−⇀↽−B(g) Kp=7.80×10−5
at 500 K If a sample of A(g) at 5.40 a...


Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Questions




History, 04.02.2020 08:01



Mathematics, 04.02.2020 08:01

English, 04.02.2020 08:01

History, 04.02.2020 08:01

History, 04.02.2020 08:01





Biology, 04.02.2020 08:01

Geography, 04.02.2020 08:01