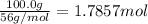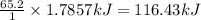Chemistry, 11.03.2020 22:59 isabellacampos4586
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following reaction. CaO (s) + H2O (l) → Ca(OH)2 (s) ΔH = - 65.2 kJ How much heat is released when 100.0 g of calcium oxide reacts with excess water?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1
You know the right answer?
Calcium oxide reacts with water to produce calcium hydroxide and 65.2 kJ of heat in the following re...
Questions

English, 11.07.2019 06:00


Arts, 11.07.2019 06:00





English, 11.07.2019 06:00


Biology, 11.07.2019 06:00

English, 11.07.2019 06:00


Mathematics, 11.07.2019 06:00


English, 11.07.2019 06:00



Mathematics, 11.07.2019 06:00


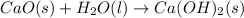 , ΔH = -65.2 kJ
, ΔH = -65.2 kJ