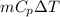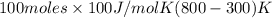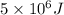Chemistry, 11.03.2020 22:56 skyhighozzie
A well-insulated, closed device claims to be able to compress 100 mol of propylene, acting as a SoaveRedlich-Kwong gas and with C ∗ P = 100 J/(mol·K), from 300 K and 2 m3 to 800 K and 0.02 m3 by using less than 5 MJ of work. Is this possible?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
A well-insulated, closed device claims to be able to compress 100 mol of propylene, acting as a Soav...
Questions

Biology, 02.06.2021 23:00



Mathematics, 02.06.2021 23:00





Arts, 02.06.2021 23:00





Biology, 02.06.2021 23:00

Mathematics, 02.06.2021 23:00





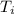 = 300 K
= 300 K
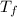 = 800 K,
= 800 K, 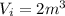
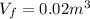 ,
, 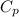 of propylene = 100 J/mol
of propylene = 100 J/mol