
Chemistry, 11.03.2020 22:56 amberpetty4288
The value of Ka for acetic acid , CH3COOH , is 1.80×10-5 . Write the equation for the reaction that goes with this equilibrium constant. (Use H3O+ instead of H+.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

You know the right answer?
The value of Ka for acetic acid , CH3COOH , is 1.80×10-5 . Write the equation for the reaction that...
Questions


Mathematics, 25.03.2020 02:14

Mathematics, 25.03.2020 02:14






Mathematics, 25.03.2020 02:14











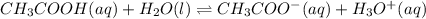
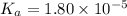
![K_c=\frac{[CH_3COO^-][H_3O^+]}{[CH-3COOH][H_2O]}](/tpl/images/0543/5879/7c275.png)
![K_a=K_c\times [H_2O]=\frac{[CH_3COO^-][H_3O^+]}{[CH-3COOH]}](/tpl/images/0543/5879/125a5.png)
![[H_2O]=1](/tpl/images/0543/5879/b8579.png)
![K_a=\frac{[CH_3COO^-][H_3O^+]}{[CH-3COOH]}](/tpl/images/0543/5879/e4df0.png)


